WATER SOLVE
WATER MEAN LIFE.
Water is the basis of life and essential for all life and processes we know.
BSE has been designs and constructs many water treatment systems incorporating de-ionization. These systems may produce Ultra Pure Water (UPW) for the semiconductor industries, in which ionic contamination is unacceptable, even at extremely low levels. While other industries describe the water they use as PW, UPW and WFI water quality standards vary widely.
Lower resistivity applications, such as pharmaceuticals and renal dialysis require Purified Water (PW) and Water For Injection (WFI).
PRETREATMENT: PRE-FILTRATION & SOFTENING
The pretreatment includes all the process steps to prepare the water to feed the final water treatment steps that could be Reverse osmosis, Elettrodeionization (EDI), Ultrafiltration and if Water for Injection (WFI) is required distillation.
The pretreatment is very important because if not adequate could cause problems to the final purification step.
PURIFIED WATER (PW)
Reverse osmosis (RO) is a technology based on a semi-permeable membrane that is permeable to the water, but impermeable to other substances as salts, endotoxins and bacteria. Reverse osmosis membrane salt removal is over (>99, 5%), the double pass RO configuration is able to guarantee with high reliability the compliance with the Pharmacopoeia requirements.

Our RO package is “tailor made” to meet the different specificity of our customers’ requirements, or starting from customer room lay out to have the best installation solution. Our Reverse Osmosis system has been successfully validated to comply with international regulations and guidelines in many industries such as cosmetic, pharmaceutical, high technology R&D center…
The PW system has been meeting the USP and European Pharmacopoeia requirements and other regulations:
ULTRA PURE WATER (UPW)
Our UPW skids and systems are completely customizable with endless options for the Pharmaceutical and Semiconductor industries.

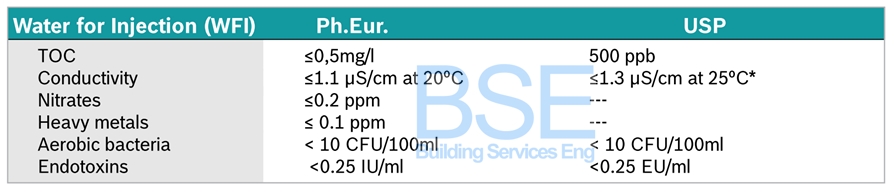
WATER FOR INJECTION (WFI)
Water for Injection (WFI) is a globally defined pharmacopeia quality standard around conductivity, total organic carbon (TOC), bacteria and bacterial endotoxin for water that is used for diluting substances in the manufacturing of parenteral and ophthalmic products, as well as the final rinsing of packaging. According to EU/US Pharmacopeia, WFI can be produced by multiple effect distillation, vapor compression distillation and membrane-based systems.